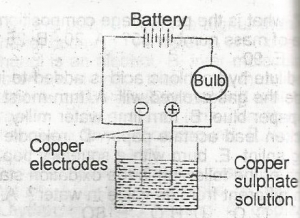
The bulb lights and copper is deposited at both electrodes
the bulb lights and copper is deposited at the anode and disappears from the cathode
the bulb lights and copper is deposited at the cathode and oxygen liberated at the anode
the bulb lights and copper is deposited at the cathode and disappears from the anode
the bulb lights but no additional changes are observed
Correct answer is D
No explanation has been provided for this answer.
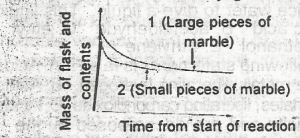
the initial slope of the curve is steepest and this occur when small pieces of marble are used and is due to a small surface area of reactant
the occur when large pieces of marble are used and is due to a small surface area of reactant
the initial slope of the curve is steepest and this occurs when small pieces of marble are used and is due to a large surface area of reactant
the initial slope of the curve is steepest and this occur when large surface area of reactant
the initial slope of the curve is least steep and this occur when small pieces of marble are used and is due to the increased surface area of the reactant
Correct answer is C
No explanation has been provided for this answer.
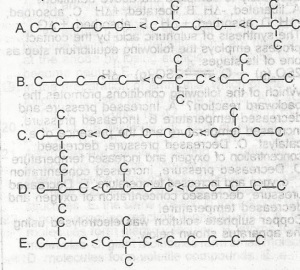
A
B
C
D
E
Correct answer is D
The boiling points of each of the compounds:
Pentane - 36.1°C ; 2- methylbutane - 27.8°C ; dimethylpropane - 10°C.
Therefore, the order of boiling point in ascending order is Dimethylpropane < 2-methylbutane < pentane.
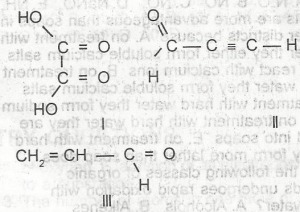
Which of the following compounds will take up the molecules of bromine?
l
ll
lll
l and ll
l and lll
Correct answer is B
No explanation has been provided for this answer.
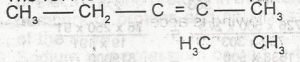
The IUPAC name for the compound is
2,3-dimethylpent-2,3-ene
2, 3- dimethylpent-2-ene
3, 4-dimethylpent-3-ene
3,4-dimethylpentene
3,4-dimethlhept-2-ene
Correct answer is B
The IUPAC name of the given compound(carefully examined) is 2,3 - dimethyl pent - 2 - ene.
JAMB Subjects
Aptitude Tests