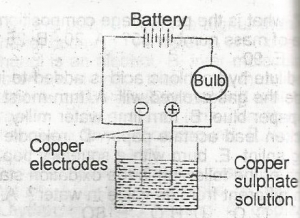
Copper sulphate solution was electrolysed using the apparatus show above. Which of the following changes are observed?
The bulb lights and copper is deposited at both electrodes
the bulb lights and copper is deposited at the anode and disappears from the cathode
the bulb lights and copper is deposited at the cathode and oxygen liberated at the anode
the bulb lights and copper is deposited at the cathode and disappears from the anode
the bulb lights but no additional changes are observed
Correct answer is D
No explanation has been provided for this answer.
Similar Questions
Which of these reagents can confirm the presence of a triple bond? ...
What is the atomic number of Tellurium? ...
The gas that is liberated when iron is heated with concentrated tetraoxosulphate (VI)acid is ...
Ethanol can easily be produced by ...
When cathode rays are deflected onto the electrode of an electrometer, the instrument becomes? ...
Which of the following compound is used for removing impurities from bauxite? ...
Which of the following statements about phosphorus is NOT correct? ...
Which of the following is a form of calcium carbonate? I. limestone II. marble III. chalk Iv. ...