Which of the following is the correct order in the above ...
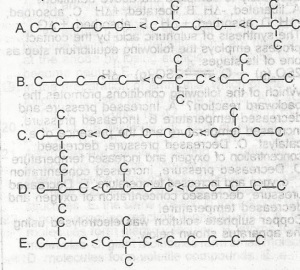
Which of the following is the correct order in the above diagram increasing boiling point, of the isomeric C5H12 compounds?
A
B
C
D
E
Correct answer is D
The boiling points of each of the compounds:
Pentane - 36.1°C ; 2- methylbutane - 27.8°C ; dimethylpropane - 10°C.
Therefore, the order of boiling point in ascending order is Dimethylpropane < 2-methylbutane < pentane.
Similar Questions
In the extraction of iron, the waste gas from the furnace is a mixture of ...
A metal that forms soluble trioxosulphate (IV) ion is ...
A suitable solvent for iodine and naphthalene is ...
The electronic configuration of an element is 1s2 2s2 2p6 3s2 3p3. Where is...
Changes in the physical in the scheme above.The letter X,Y and Z respectively represent...
Consider the following exothermic reaction 2SO2(g) + O2(g) ↔ 2SO3(g) If the temperature of the r...
The metal that liberates hydrogen from cold water in bubbles only is? ...
P(g) + Q(g) ↔ 3R(s) + S(g) ΔH is negative Which of the following will increase the yield of R?...