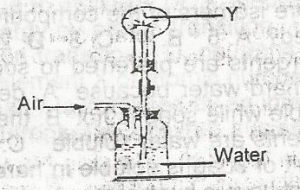
In the diagram above the gas Y could be
hydrogen chloride
oxygen
carbon (IV) oxide
chlorine
Correct answer is A
- Two gases that can be used in the study of fountain experiment is ammonia gas and hydrogen chloride gas.
- Ammonia dissolves into the water and the pressure in the container drops. As a result, more water is forced into the container from another inlet creating a fountain effect.
- All the hydrogen chloride in the flask dissolves in the water drops from the pipette, creating a vacuum in the flask. Water is then sucked up at great speed from the bowl, creating a spectacular 'fountain' in the flask.
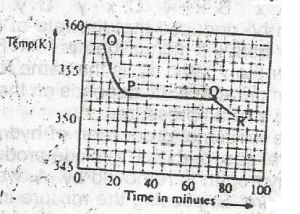
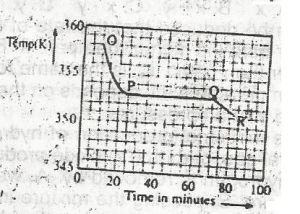
liquid sate
solid/liquid state
solid state
gaseous state
Correct answer is A
No explanation has been provided for this answer.
a mixture of salts
a hydrated salt
an ionic salt
a pure compound
Correct answer is D
No explanation has been provided for this answer.
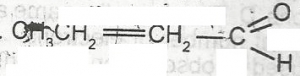
The functional group represented in the compound above is?
alkanol
alkanal
alkanone
alanoate
Correct answer is B
The carbonyl group in tha alkanal (aldehyde) family is always at the end of a carbon chain
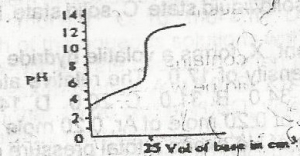
The option above shows the PH changes for the titration of a
strong acid versus strong base
weak acid versus strong base
strong acid versus weak base
weak acid versus weak base
Correct answer is B
Option B is a graph of weak a acid and strong base
JAMB Subjects
Aptitude Tests