Use the graph above to answer this question. A sample, X,...
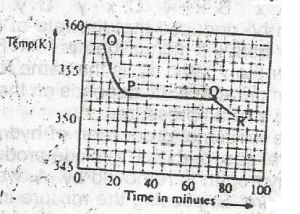
Use the graph above to answer this question. A sample, X, solid at room temperature, was melted, heated to a temperature of 358 K and allowed to cool as shown in OPQR.The section OP suggests that X is in the
liquid sate
solid/liquid state
solid state
gaseous state
Correct answer is A
No explanation has been provided for this answer.
Similar Questions
Who proposed the planetary model of the atom with electrons orbiting the nucleus? ...
The pH of water is 7. What is the pH of orange juice? ...
Which of the following statements about dative bonding is not correct? ...
X + Y = Z is an equilibrium reaction. The addition of a catalyst? ...
PCL5(g)= PCL3(g) + Cl2(g) In the reaction above, a decrease in pressure will...