Use the graph above to answer this question. A sample, X,...
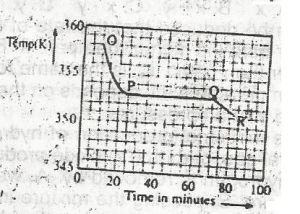
Use the graph above to answer this question. A sample, X, solid at room temperature, was melted, heated to a temperature of 358 K and allowed to cool as shown in OPQR. The section PQ indicates that X is
A.
a mixture of salts
B.
a hydrated salt
C.
an ionic salt
D.
a pure compound
Correct answer is D
No explanation has been provided for this answer.