In the diagram above the gas Y could be
...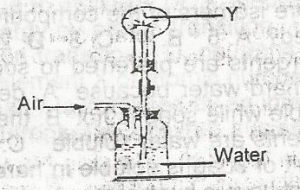
In the diagram above the gas Y could be
hydrogen chloride
oxygen
carbon (IV) oxide
chlorine
Correct answer is A
- Two gases that can be used in the study of fountain experiment is ammonia gas and hydrogen chloride gas.
- Ammonia dissolves into the water and the pressure in the container drops. As a result, more water is forced into the container from another inlet creating a fountain effect.
- All the hydrogen chloride in the flask dissolves in the water drops from the pipette, creating a vacuum in the flask. Water is then sucked up at great speed from the bowl, creating a spectacular 'fountain' in the flask.
Similar Questions
Farmlands affected by crude-oil spillage can be decontaminated by ...
The electrons of two atoms Y and Z are arranged in shells as shown above. The bond formed betwe...
The energy change which accompanies the addition of an electron to a gaseous atom is ...
A mixture of petrol and water can be separated through ...
Which of the following species does not contain a co-ordinate bond? ...
Vinegar is an aqueous solution of? ...
The solution of a sample in a tube will be identified as a chloride if it gives...
Which of the following is used as an 'anti-knock' in automobile engines? ...
A metal X forms two chlorides with the formulae XCI2 and XCI3. Where is X in the Periodic Table?...
Which of the following statements is true for strong electrolytes? ...