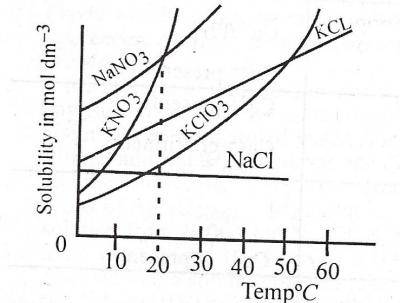
At what temperature does the solubility of \(KNO_{3}\) equal that of \(NaNO_{3}\)?
0°C
20°C
30°C
40°C
Correct answer is B
From the graph, check for their point of intersection and trace it down to the temperature axis.
Pure water can be made to boil at a temperature lower than 100°C by
reducing its quantity
decreasing the external pressure
distilling it
increasing the external pressure
Correct answer is B
The easiest and most drastic way to change the boiling point is by tampering with the pressure above your water. To lower the boiling point, you need simply to decrease its external pressure.
0.490g
0.049g
0.245g
0.0245g
Correct answer is C
Using
n = cv where n = no of mole, c = molar concentration(mol/dm3) and v = volume( dm3)
volume = 500\(cm^{3}\) = 0.5dm3, c = 0.005\(moldm^{-3}\)
number of mole(n) = c x v = 0.5 X 0.005 = 0.0025mol.
but n = \(\frac{mass}{ molar mass}\) = 0.0025 = \(\frac{mass}{ 98}\) (where 98g/mol is the molar mass of H2SO4)
mass of H2SO4 = 0.0025 x 98 = 0.245g.
a decrease in pressure
an increase in pressure
a decrease in temperature
an introduction of a positive catalyst
Correct answer is A
The equilibrium position is shifted to the left with a decrease in pressure in this system because the number of gaseous molecules on the right is less. (Le-Chatelier's principle).
Ethene is produced from ethanol by
decomposition
hydrolysis
ozonolysis
dehydration
Correct answer is D
Ethene can be gotten from the dehydration of ethanol in the presence of excess \(H_{2}SO_{4}\) or \(H_{3}PO_{4}\).
WAEC Subjects
Aptitude Tests