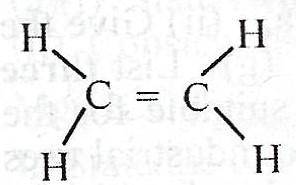
What is the total number of shared pair of electrons in the compound above?
5
8
10
12
Correct answer is D
Each double bond has two pairs of valence electrons shared to form a covalent bonding. Also, every C-H bond, has 2 valence elctrons involved. Drawing the Lewis structure, you see that the above compound has 12 valence electrons shared.
The atomic number of an isotope of hydrogen is equal to its mass number because it
has a totally filled valence shell
has a high charge to mass ratio
does not contain neutrons
exhibits isotopy
Correct answer is C
No explanation has been provided for this answer.
Which of the following electron configurations represents the transition element Chromium 24Cr?
1s22s22p63s23p64s23d4
1s22s22p63s23p63d6
1s22s22p63s23d44s1
1s22s22p63s23p64s13d5
Correct answer is D
Electronic configuration of Chromium: 1s22s22p63s23p64s13d5
WAEC Subjects
Aptitude Tests