What is the total number of shared pair of electrons in t...
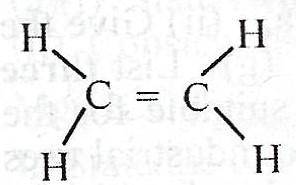
What is the total number of shared pair of electrons in the compound above?
5
8
10
12
Correct answer is D
Each double bond has two pairs of valence electrons shared to form a covalent bonding. Also, every C-H bond, has 2 valence elctrons involved. Drawing the Lewis structure, you see that the above compound has 12 valence electrons shared.
Similar Questions
The alkyl group is represented by the general formula ...
A phenomenon where an element exist in different forms in the same physical state is known as ...
The oxidation number of iodine in KIO3 is...
Starch could be converted to glucose by the process of ...
An element used in the production of matches is ...
In the experiment above, X is a mixture of nitrogen, carbon (IV) oxide and ...
Which or the following products could be formed during incomplete combustion of a hydrocarbon. ...