How many atoms are present in 6.0g of magnesium? [Mg = 24, N.A = 6.02 x 10 \(^{23}\)mol]
1.20 x 10\(^{22}\)
2.4 x 10\(^{22}\)
1.51 x 10\(^{22}\)
3.02 x 10\(^{22}\)
Correct answer is C
No explanation has been provided for this answer.
0.40 moldm\(^{-3}\)
0.50 moldm\(^{-3}\)
0.05 moldm\(^{-3}\)
0.30 moldm\(^{-3}\)
Correct answer is B
No explanation has been provided for this answer.
Zn
Ca
Al
Pb
Correct answer is A
No explanation has been provided for this answer.
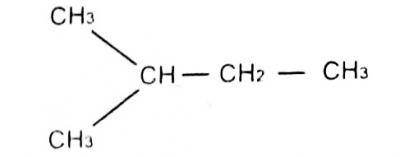
1-methyl pentane
3-methylbutane
2-methylbutane
1-dimethyl propane
Correct answer is C
The longest carbon chain is butane, with one substituent attached at carbon number 2
therefore the compound is named 2-methyl butane
lead oxide
Magnesium oxide
Copper oxide
Tin oxide
Correct answer is B
The oxides of Potassium, Sodium, Calcium, and Magnesium are not reduced when they react with carbon and hydrogen.
JAMB Subjects
Aptitude Tests