The hybridization in the compound \(CH_3 - CH_2 - C \equiv H\) is
sp\(^3\) and sp
sp
sp\(^2\)
sp\(^3\) and sp\(^2\)
Correct answer is A
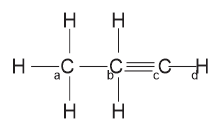
The hybridization in a and b is sp\(^3\) hybridization while in c and d is sp hybridization.
Z\(_3\)(SO\(_4\))\(_2\)
ZSO\(_4\)
Z\(_2\) SO\(_4\)
Z\(_2\)(SO\(_4\))\(_3\)
Correct answer is D
Z = 1s\(^2\) 2s\(^2\) 2p\(^6\) 3s\(^2\) 3p\(^1\)
\(\therefore\) We have Z\(^{3+}\) and SO\(_4 ^{2-}\)
The reaction : Z\(^{3+}\) + SO\(_4 ^{2-}\) \(\to\) Z\(_2\)(SO\(_4\))\(_3\).
Which of the following could not be alkane?
C\(_4\)H\(_{10}\)
C\(_5\)H\(_{12}\)
C\(_7\)H\(_{14}\)
C\(_8\)H\(_{18}\)
Correct answer is C
No explanation has been provided for this answer.
The two ions responsible for hardness in water are
Ca\(^{2+}\) and / or Mg\(^{2+}\)
K\(^+\) and / or Mg\(^{2+}\)
Ca\(^{2+}\) and / or Li\(^+\)
Ca\(^{2+}\) and / or Na\(^+\)
Correct answer is A
No explanation has been provided for this answer.
1.7 litres
0.420 litres
0.588 litres
2.35 litres
Correct answer is C
No explanation has been provided for this answer.
JAMB Subjects
Aptitude Tests