A suitable solvent for iodine and naphthalene is
carbon (IV) sulphide
ethanol
water
benzene
Correct answer is B
No explanation has been provided for this answer.
Hardness of water is mainly due to the presence of
calcium hydroxide or magnesium hydroxide
calcium trioxocarbonate (IV) or calcium tetraoxosulphate (VI)
sodium hydroxide or magnesium hydroxide
calcium chloride or sodium chloride salts
Correct answer is B
Salts of Calcium and Magnesium are responsible for hardness of water. Bicarbonates, Carbonates, Chlorides and Sulfates of Calcium and Magnesium causes temporary and permanent hardness.
What type of bond exits between an element X with atomic number 12 and Y with atomic number 17?
Electrovalent
Metallic
Covalent
Dative
Correct answer is A
No explanation has been provided for this answer.
The importance of sodium aluminate (III) in the treatment of water is to
cause coagulation
neutralize acidity
prevent goitre and tooth decay
kill germs
Correct answer is A
No explanation has been provided for this answer.
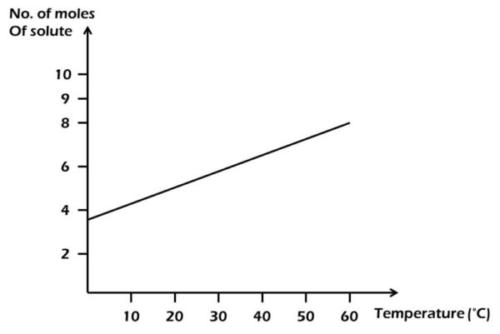
0.10 mole
0.20mole
0.01 mole
0.02 mole
Correct answer is B
From the diagram,
55°C = 7 moles; 40°C = 6 moles.
Amount of solute deposited = 7 - 6 = 1 mole.
1000 cm\(^3\) = 1 mole
200 cm\(^3\) = x
x = \(\frac{200 \times 1}{1000}\)
= 0.20 mole.
JAMB Subjects
Aptitude Tests