From the diagram above, find the amount of solute deposit...
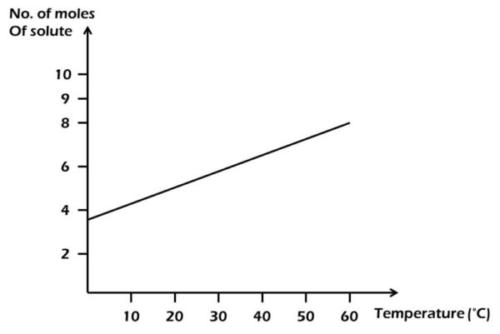
From the diagram above, find the amount of solute deposited when 200 cm33 of the solution is cooled from 55°C to 40°C.
A.
0.10 mole
B.
0.20mole
C.
0.01 mole
D.
0.02 mole
Correct answer is B
From the diagram,
55°C = 7 moles; 40°C = 6 moles.
Amount of solute deposited = 7 - 6 = 1 mole.
1000 cm3 = 1 mole
200 cm3 = x
x = 200×11000
= 0.20 mole.
Similar Questions
n a chemical reaction, the reacting species possess energy of motion known as ...
The gas that can be collected by downward displacement of air is ...
A compound that will NOT produce oxygen on heating is? ...
The species with no electron in the 3d-orbital is ...
The electronic configuration of an element is 1s2 2s2 2p6 3s2 3p3. Where is...