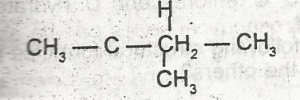
The I.U.P.A.C name for the compound is
isopropylethene
acetylene
3- methylbutane
2-methylbutane
5-methylpentane
Correct answer is D
No explanation has been provided for this answer.
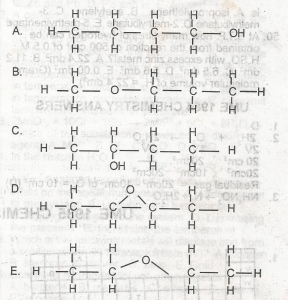
A
B
C
D
E
Correct answer is D
Isomers have the same molecular structures(but different structures). so whatever the structures are, they must contain the same amount of carbon, the same amount of hydrogen, and the same amount of oxygen as in this case. Just count the number of hydrogens (including the hydrogen in OH). you should get 10 hydrogens from A, B, C, and E. in total, you should have C4H10O. options A, B, C, and E have this in common. but the option D has only C4H8O.
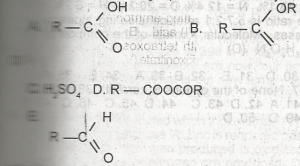
USE THE DIAGRAM ABOVE TO ANSWER THIS QUESTION. Which of the following represents a carboxylic acid?
A
B
C
D
E
Correct answer is A
A carboxylic acid is an organic acid that contains a carboxyl group attached to an R-group. The general formula of a carboxylic acid is R−COOH or R−CO₂H, with R referring to the alkyl, alkenyl, aryl, or other group.
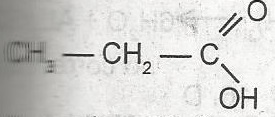
The name of the compound above is
acetic acid
propanal
propanol
ethanoic acid
propanoic acid
Correct answer is E
the compound is propanoic acid, it contains the carboxyl group COOH.
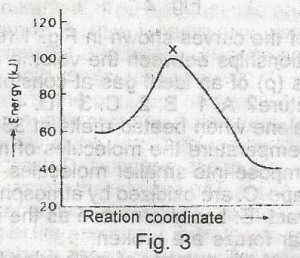
spontaneous
isothermal
adiabatic
exothermic
endothermic
Correct answer is D
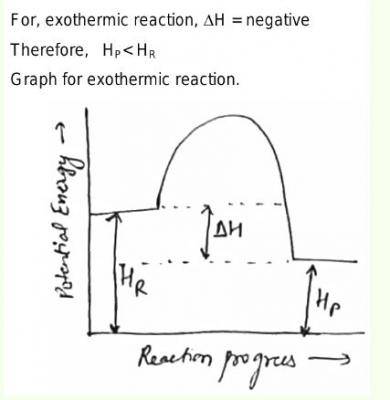
No explanation has been provided for this answer.
JAMB Subjects
Aptitude Tests