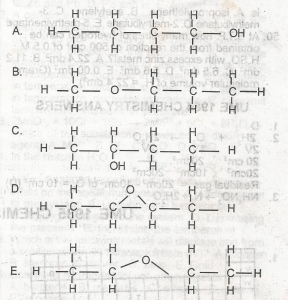
Use the diagram above to answer this question Which of the following structural formula is NOT isomeric with the others?
A
B
C
D
E
Correct answer is D
Isomers have the same molecular structures(but different structures). so whatever the structures are, they must contain the same amount of carbon, the same amount of hydrogen, and the same amount of oxygen as in this case. Just count the number of hydrogens (including the hydrogen in OH). you should get 10 hydrogens from A, B, C, and E. in total, you should have C4H10O. options A, B, C, and E have this in common. but the option D has only C4H8O.
Similar Questions
The bond which joins two ethanoic acid molecules in the liquid state is...
Benzene produces more soot than ethene on burning because benzene ...
Which of the following will change when a catalyst is added to a chemical reaction? ...
Hardness of water is caused by the presence of ions of ...
Oxygen-demanding waste are considered to be a water pollutant because they? ...
Detergents are better than soaps for washing in hard water because ...
The IUPAC name for the compound is...
Hydrogen is readily released when dilute hydrochloric acid reacts with ...