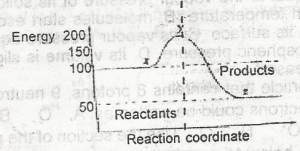
The ∆H for the reaction represented by the energy profile above is
-100 kJ mol--1
+ 100 kJ mol-1
+50 kJ mol-1
-50 kJ mol-1
Correct answer is D
No explanation has been provided for this answer.
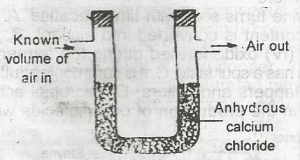
The set-up above would be useful for determining the amount of
oxygen in air
water vapour in air
CO2 in air
arygen in air
Correct answer is B
No explanation has been provided for this answer.
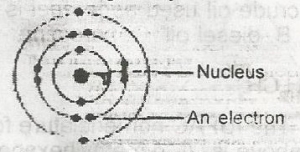
The diagram above represents an atom of
magnesium
helium
chlorine
neon
Correct answer is A
The diagram refers to an atom with electronic configuration 2,8,2 hence the atomic number = 12. From the periodic table, we have the atom to be Mg (Magnesium).
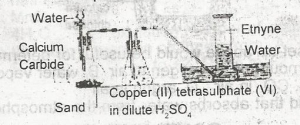
The function of the copper (ll)tetraoxosulphate (IV) in dilute H2SO4 in the figure above is to
dry the gas
absorb phosphine impurity
absorb ethene impurity
from an acetylide with ethyne
Correct answer is B
No explanation has been provided for this answer.
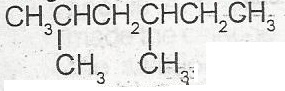
The IUPAC nomenclature for the compound above is
dimethlhexane
3,5 dimethylhexane
1.1 dmethyl, 3 methylpentane
2.4 dimethylhexane
Correct answer is D
No explanation has been provided for this answer.
JAMB Subjects
Aptitude Tests