The ∆H for the reaction represented by the energy profi...
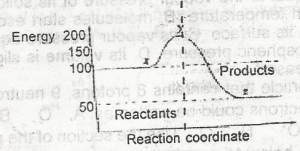
The ∆H for the reaction represented by the energy profile above is
-100 kJ mol--1
+ 100 kJ mol-1
+50 kJ mol-1
-50 kJ mol-1
Correct answer is D
No explanation has been provided for this answer.
Similar Questions
Which of the following gases is absorbed from the air dun photosynthesis? ...
It can be deduced from the vapour pressure curves above that...
A good drying agent should be ...
Which of the following compounds is covalent? ...
mE + nF → pG + qH. In the equation above, the equilibrium constant is given by? ...
How many isomers has C 3H 6CI 2?...
Which of the following groups contains entirely linear molecules? ...
The relative atomic mass of a naturally occurring lithium consisting of 90% 73Li and \(^6 _3\...