NaCl and KNO\(_{3}\)
KCL and NaNO\(_{3}\)
K\(_{2}\)SO\(_{4}\) and BaCl\(_{2}\)
NH\(_{4}\)NO\(_{3}\) and CO\(_{3}\)
Correct answer is C
when potassium sulfate is mixed with barium chloride a white precipitate of barium sulfate will be form. barium sulfate is slightly soluble in water, so it will precipitate out as a solid
BaCl2 + K2SO4 → BaSO4 + 2KCl
note; all substances are aqueous except BaSO4 which is solid
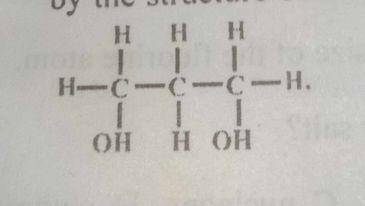
The alkanol represented by the structure above is
primary and dihydric
secondary and monohydric
tertiary and dihydric
secondary and dihydric
Correct answer is A
It is primary alcohol because it has only one alkyl group and dihydric because it has two hydroxyl groups
Give the common name for the following compound (CH\(_{3}\))\(_{2}\) CH CH\(_{2}\) -Br
Isobutyl bromide
Methyl bromide
propyl bromide
Butyl bromide
Correct answer is A
Common name : Isobutyl bromide
IUPAC name : 1-Bromo-2-methylpropane
Boiling point : 102°C
Formula : C\(_4\)H\(_9\)Br
An example of a biodegradable pollutant is?
plastic
sewage
carbon (II) oxide
hydrogen sulphide
Correct answer is B
examples of biodegradable
- Food waste.
- Paper waste.
- Human waste.
- manure.
- sewage.
- Sewage sludge
- Glass, metals, electronic devices, computer parts, batteries, medical waste, plastic bags, plastic bottles, tetra packs, and carbon paper are a few examples of non- biodegradable materials.
I and Il only
I, II and IV only
II, IIl and IV only
I, II, III and IV
Correct answer is C
- CaCl2 is an ionic compound owing to the large electronegative difference between the calcium atom and chlorine atom, which is greater than 2.0. In calcium chloride, the calcium atom donates its two electrons and become cation whereas each chlorine atom gain one electron, donated by Calcium, and get a negative charge.
- Ionization energies are always positive numbers, because energy must be supplied (an endothermic energy change) to separate electrons from atoms.
- Chlorine has a high ionization energy
- Chlorine has high electron affinity among the elements. Its high affinity can be attributed to its large atomic radius, or size. Because chlorine's outermost orbital is 3p, its electrons have a large amount of space to share with an incoming electron.
WAEC Subjects
Aptitude Tests