P and T are increased
both T and P are decreased
T is increased and P is decreased
T is decreased and P is increased
Correct answer is C
No explanation has been provided for this answer.
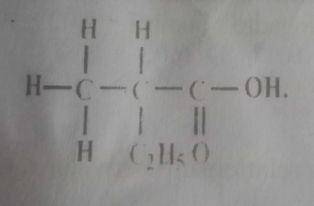
Consider the structure above How many carbon atoms does the parent chain contain?
5
4
3
2
Correct answer is B
Going by the longest continuous carbon chain, the parent chain contains 4 carbon atoms (2- methyl butanoic acid)
When CuSO\(_{4(aq)}\) is added to Pb(NO\(_{3}\))\(_{2(aq)}\)--------
there would be no visible change
a blue precipitate would be formed
the resulting solution would become colourless
a white precipitate would be formed
Correct answer is D
No explanation has been provided for this answer.
blue precipitate is formed which is soluble in excess ammonia
brick red precipitate is produced which is insoluble in excess ammonia
which precipitate is formed which is excess in ammonia
green precipitate is formed which is insoluble in excess ammonia
Correct answer is A
No explanation has been provided for this answer.
The basic property of salts used as drying agents is by?
efforescence
high melting point
hygroscopy
low solubility
Correct answer is C
Hygroscopic substances are used to keep products dry or to remove water from an area. They are commonly used in desiccators
Some common examples of hygroscopic substances include: Sodium chloride. Zinc chloride. Calcium chloride
WAEC Subjects
Aptitude Tests