The following statements are correct except
energy is released when liquids change to solids.
carbon atoms in gaseous methane are further apart than those in solid diamond.
there is large decrease in the volume of a solid metal when pressure is applied to it.
particles move faster in the gaseous state than in the liquid state.
Correct answer is C
In general, solids are relatively incompressible compared to liquids and gases. When pressure is applied to a solid, there is typically only a small change in volume. Solids have tightly packed particles with strong intermolecular forces, making them less responsive to changes in pressure. Liquids and gases, on the other hand, can undergo larger volume changes in response to pressure.
40
120
65
30
Correct answer is D
Molar mass of C2H4O2,= (12 × 2) + (1 × 4) + (16 × 2)= 60 g/mol
Vapour density = RMM/2
=60/2=30
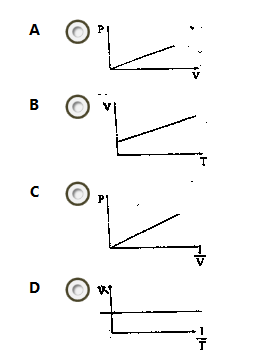
Which of the following sketches is a graphical representation of Boyle's law?
A
B
C
D
Correct answer is C
Boyles law states that the volume of a given mass of gas is inversely proportional to pressure at constant temperature.
Which of the following scientists formulated the law of conservation of mass?
A. Lavoisier
J. Dalton
R. Boyle
J. Proust
Correct answer is A
Law of conservation of mass was given by Antoine Lavoisier in 1744. According to this law, "Matter can neither be created nor destroyed in a chemical reaction".
The first definition of an element was made by
J. Dalton.
A. Lavoisier.
R. Boyle.
J. J. Thompson.
Correct answer is C
The word element was first given by Rebert Boyle. It is defined as the simplest chemical substance that cannot be broken down during a chemical reaction
WAEC Subjects
Aptitude Tests