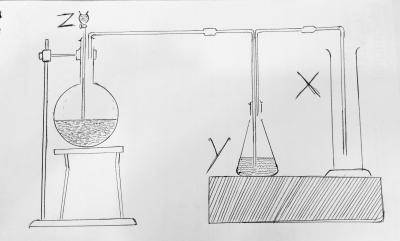
fused CaO
H2O
NaOH
Concentrated H2SO4
Correct answer is D
In the preparation of sulphur dioxide by the action of dilute acids on sulphates and bisulphites. conc H2SO4 helps to release SO2 from the mixture.
The setup represents the production of sulphur dioxide
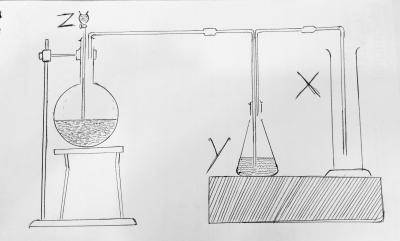
SO3
SO2
S
H2S
Correct answer is B
The setup represents the production of sulfur dioxide. And the cylinder marked X is SO2
Cu2S(g) + O2(g) → 2Cu + SO2(g)
What is the change in the oxidation number of copper in the reaction?
0 to + 2
0 to + 1
+ 1 to 0
+ 2 to + 1
Correct answer is C
In the reactant;
Cu2S
2 Cu - 2(1) = 0
2 Cu = 2
Cu = \(\frac{2}{2}\)
Cu = +1
In the product, Cu
Cu = O
The oxidation number of Cu in Cu2S and Cu respectively is +1 and 0 respectively
The alkyl group is represented by the general formula
CnH2n
CnH2n − 2
CnH2n + 1
CnH2n + 2
Correct answer is C
Alkyl group has the general formula CnH2n + 1.
This formed when one hydrogen is removed from the alkane family. It has the general formula CnH2n + 1
The enzyme used in the hydrolysis of starch to dextrin and maltose is?
lipase
amylase
invertase
zymase
Correct answer is B
Starch is hydrolyzed by dilute acids to yield a mixture of dextrin (a shorter chain intermediate product), disaccharides (mainly maltose) and glucose. Further hydrolysis will eventually give glucose. Hydrolysis of starch to dextrin and maltose can also be brought about by the enzyme amylase present in saliva and in malt.
JAMB Subjects
Aptitude Tests