The carbon atoms on ethane are
sp2 hybridized
sp3 hybridized
sp4 hybridized
sp hybridized
Correct answer is B
Alkane's family are sp3 hybridized
0.1
0.5
2.0
4.0
Correct answer is C
The rate of dimension of a gas inversely proportional to the square root of its molecular mass or its density, which is Graham's Law of diffusion of gas.
R ∝ \(\frac{1}{\sqrt{Mm}}\) or R ∝ \(\frac{1}{\sqrt{D}}\)
Dx = 0.5gdm-3, Dy = 2gdm-3
R= \(\frac{K}{\sqrt{D}}\)
R\(\sqrt{D}\) = k
R1\(\sqrt{D_1}\) = R1\(\sqrt{D_2}\)
Rx\(\sqrt{D_x}\) = Ry\(\sqrt{D_y}\)
\(\frac{R_x}{R_y}\) = \(\frac{\sqrt{D_y}}{\sqrt{D_x}}\)
= \(\frac{\sqrt{2}}{\sqrt{0.5}}\)
= 2.0
The reddish–brown rust on iron roofing sheets consists of
Fe3 + (H2O)6
FeO.H2O
Fe2O3.XH2O
Fe3O4.2H22O
Correct answer is C
Iron [Fe] reacts with H2 in the presence of oxygen to form a rust.
4Fe + 3O2 → 2 Fe2O2
Fe2O3 + H2O → Fe2O3.H2O
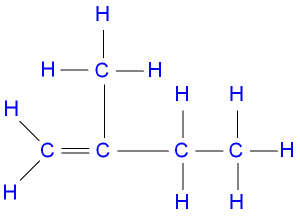
The IUPAC nomenclature of the structure above is
3-methybut-3-ene
2-methylbut-1-ene
2-ethylprop-1-ene
2-methylbut-2-ene
Correct answer is B
CH2CH2 - CCH3CH2
Start the numbering from the terminal carbon.
0.300g
0.250g
0.2242g
0.448g
Correct answer is D
M = \(\frac{\text{Molar mass × Quantity of Electricity}}{\text{96500 × no of charge}}\)
= \(\frac{MmIT}{96500n}\)
Copper II Chloride = CuCl2
CuCl2 → Cu2+ + 2Cl2
Mass of compound deposited = \(\frac{\text{Molar mass × Quantity of Electricity}}{\text{96500 × no of charge}}\)
Q = IT
I = 0.5A
T = 45 × 60
T = 2700s
Q = 0.5 × 2700
= 1350c
Molarmass = 64gmol-1
no of charge = + 2
Mass = \(\frac{64 \times 1350}{96500 \times 2}\)
Mass = 0.448g
JAMB Subjects
Aptitude Tests