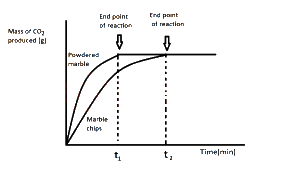
The graph above demonstrate the effect of?
surface area on the rate of reaction
catalyst on the rate of reaction
pressure on the rate reaction
concentration on the rate of reaction
Correct answer is A
No explanation has been provided for this answer.
lowering the pressure of the reaction
increasing the surface area of the reactant
increase the rate of the reaction
lowering the energy barrier of the reaction
Correct answer is C
- The presence of MnO2 increase the rate of the reaction by providing a new reaction path / making more particles possess kinetic energy greater then the activation energy.
If a reaction is exothermic and there is a great disorder, it means that?
the reaction is static
the reaction is in a state of equilibrium
there will be a large increase in free energy
there will be a large decrease in free energy
Correct answer is D
If a reaction is exothermic and there is a great disorder for a spontaneous reaction to occur, a large decrease in free energy is evident.
JAMB Subjects
Aptitude Tests