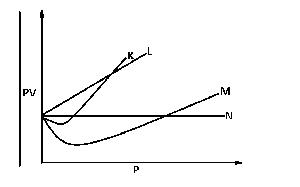
From the diagram above, an ideal can be represented by
M
N
K
L
Correct answer is B
No explanation has been provided for this answer.
Which of the following properties is NOT peculiar to matter?
kinetic energy of particles increase from solid to gas
Random motion of particles increases from liquid to gas
Orderliness of particles increases from gas to liquid
Random motion of particles increases from gas to solid
Correct answer is D
No explanation has been provided for this answer.
0.40 mol dm-3
0.50 mol dm-3
0.05 mol dm-3
0.30 mol dm-3
Correct answer is B
No explanation has been provided for this answer.
The compound that is used as an anesthetic is?
CCl4
CH Cl3
CH2Cl2
CH3Cl
Correct answer is B
- Chloroform / tricholoromethane ( CHCl\(_3\) ) is a potent anaesthetic agent.
- Though Chloroform is no longer used as an anaesthetic for several reasons, the most important of which is the relatively high risk of complications, including possible heart failure
Benzene
Alkynes
Alkenes
Alkanes
Correct answer is D
Bromine water is an orange solution of bromine. It becomes colorless when it is shaken with an alkene. Alkenes can decolorize bromine water, but alkanes cannot.
X is ethene which decolorizes bromine water showing that ethene is unsaturated.
Y is an alkane.
JAMB Subjects
Aptitude Tests