Which of the following statements is true about 2-methylpropane and butane
They are not members of the same homologous series
They have the same boiling point
They have different number of carbon atoms
They have the same chemical properties
Correct answer is D
No explanation has been provided for this answer.
alkanol
alkane
alkanal
alkanone
Correct answer is C
No explanation has been provided for this answer.
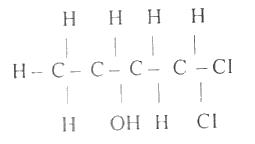
The functional groups present in the compound above are
alkene and halo-group
hydroxyl and chloro-group
alkene and chloro-group
hydroxyl and halo-group
Correct answer is D
No explanation has been provided for this answer.
The number of isomers that can be obtained from C4H10 is
3
4
1
2
Correct answer is D
No explanation has been provided for this answer.
propanol
butanol
methanol
ethanol
Correct answer is D
No explanation has been provided for this answer.
JAMB Subjects
Aptitude Tests