sulphur (VI) trioxide
tetraoxosulphate (VI) acid
trioxosulphate (IV) acid
dioxosulphate (II) acid
hydrogen sulphide
Correct answer is C
No explanation has been provided for this answer.
How many isomeric forms are there for the molecular formula C3H6Br2?
1
2
3
4
5
Correct answer is D
No explanation has been provided for this answer.
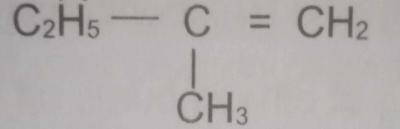
The correct name of the compound with the above structural formula is
2-methylbut -1-ene
2-methylbut -2-ene
2-methylbutane
2-ethylprop-1 -ene
2-ethylprop-2-ene
Correct answer is A
C2H5 - CH=CH2
CH3
the compound above is numbered from right to left and is named 2 - methyl but - 1 - ene.
iron is in the metallic from while the copper is in the ionic from
the atomic weight of copper is greater than that of iron
copper is greater than iron
copper metal has more electrons than iron metal
iron is higher in the electrochemical series than copper
Correct answer is E
No explanation has been provided for this answer.
1 only
2 only
3 only
1 and 3
2 and 4
Correct answer is D
No explanation has been provided for this answer.
JAMB Subjects
Aptitude Tests