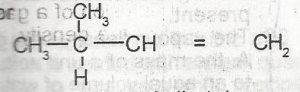
2-methylbut-3-ene
2-methylbut-4-ene
3-methylbut-2-ene
3-methylbut-1-ene
3-methylpent-1-ene
Correct answer is D
No explanation has been provided for this answer.
NaOH solution by 70 cm3
NaOH solution, by 60 cm3
NaOH solution by 40 cm3
Al(NO3)3 solution by 20 cm3
Al(NO3)3 solution by 10 cm3
Correct answer is C
No explanation has been provided for this answer.
1.35g
1.00g
0.70g
0.65g
0.00g
Correct answer is A
\(Zn + H_2 SO_4 → ZnSO_4 + H_2\)
1 mole of Zn = 1 mole \(H_2SO_4\)
no of moles of \(H_2SO_4\) = C X V = 1 X 10/1000 = 0.01moles.
0.01mole \(H_2SO_4\) = 0.01 mole Zn
mass of Zn = 0.01 x 65(given) = 0.65g
therefore, the mass of undissolved Zn = 2.00(given) - 0.65 = 1.35g.
zn + 2HCl → ZnCl2 + H2
The rate of the above reaction will be greatly increased if
the zinc is in the powered form
a greater volume of the acid is used
a smaller volume of the acid is used
the reaction vessel is immersed in an ice-bath
the zinc is in the form of pellets
Correct answer is A
No explanation has been provided for this answer.
more CuCl is formed at 40oC
more CuCl2 is formed at 10oC
Less CuCl2 is formed at 10oC
there is no change in the amount of CuCl2 formed at 40oC and10oC
more CuCl is consumed at 40oC
Correct answer is B
No explanation has been provided for this answer.
JAMB Subjects
Aptitude Tests