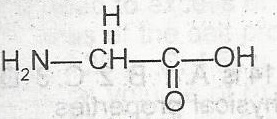
The two functional groups in the above compound are
alcohol and amine
acid and amine
aldehyde and acid
ketone and amine
Correct answer is B
No explanation has been provided for this answer.
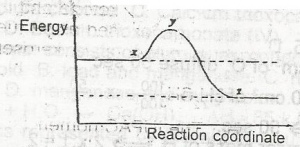
Increase in temperature
Increase in the concentration of a reactant
Addition of a catalyst
Increase in pressure
Correct answer is C
No explanation has been provided for this answer.

Cu
Fe
Ba
Zn
Correct answer is C
Elements with lower electrode potentials are most likely to be reduced and act as a reducing agent. Lower electrode potential indicates a greater tendency to accept electrons.
CaSO4
KNO3
NaCl
KCl
Correct answer is B
For many solids that are dissolved in water or any liquid, the solubility increases with a temperature rise. With an increase in kinetic energy, the solvent molecules effectively break apart the solute molecules that are held together by strong intermolecular attractions, and thereby solubility is increased.
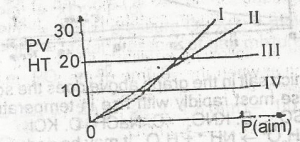
Which of the curves above represents the behaviour of 1 mole of an ideal gas?
l
ll
lll
lV
Correct answer is C
No explanation has been provided for this answer.
JAMB Subjects
Aptitude Tests