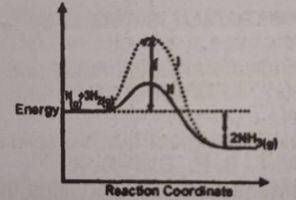
It can be deduced that the rate of the reaction?
for bath l is higher than path ll
for path ll is higher than path l
is the same for both paths at all temperatures
depends on the values of both x and y at all pressures
Correct answer is B
No explanation has been provided for this answer.
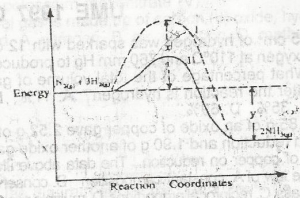
Use the diagram above to answer this question. The activation energy of the uncatalysed reaction is
X
X + Y
X - Y
Y
Correct answer is A
No explanation has been provided for this answer.
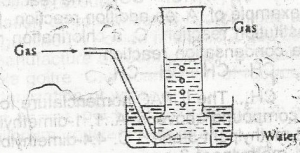
The arrangement above can be used for the collection of
sulphur (IV) oxide
ammonia
hydrogen chloride
nitrogen
Correct answer is D
This apparatus can be seen either in the preparation of Nitrogen gas from AIR or from the reduction of Copper(II) Oxide by Ammonia in which Nitrogen gas is collected in the gas jar over water.
Reaction Equation:
\(3CuO_{(s)} + 2NH_{{3}{(g)}} \to 3Cu_{(s)} + 3H_{2}O_{(l)} + N_{{2}{(g)}}\)

T
R
J
X
Correct answer is A
No explanation has been provided for this answer.

M and E
G and E
R and L
G and L
Correct answer is D
The alkali metals are found in group one while the noble gases are found in group zero or eight of the periodic table, therefore G and L belong to groups one and eight respectively.
JAMB Subjects
Aptitude Tests