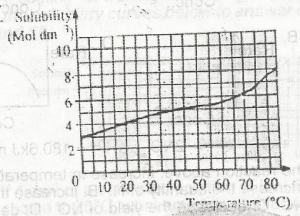
0.750 mole
0.950 mole
2.375 moles
4.750 moles
Correct answer is A
No explanation has been provided for this answer.
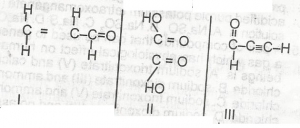
Which of the compounds above would react to take up two molecules of bromine durng bromination?
l only
lll only
l and ll only
ll and lll only
Correct answer is C
No explanation has been provided for this answer.
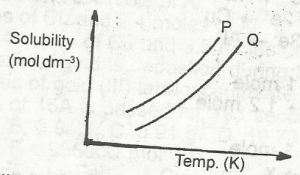
In the diagram above, the mixture of the solids P and Q can be separated by?
distillation
fractional distillation
crystallization
fractional crystallization
Correct answer is D
The process involves a series of repeated-crystallizations.
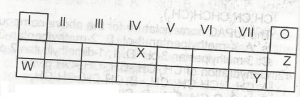
Use the table above to answer this question .The least reactive element is
W
X
Y
Z
Correct answer is D
No explanation has been provided for this answer.
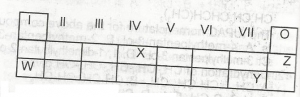
Z
Y
X
W
Correct answer is C
the element likely to participate in covalent rather than ionic bonding is X, covalent bonding is a type of bonding that involves the participants contributing an equal number of electrons into a shared pair.
JAMB Subjects
Aptitude Tests