Equilibrium is said to be attained in reversible reaction when
all the reactants have been used up
all the products have been formed
there is no further change in temperature
the rates of the forward and backward reactions are equal
the rate of formation of the products decreases with time
Correct answer is D
No explanation has been provided for this answer.
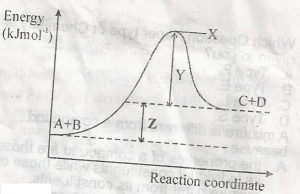
heat of reaction
activation energy
free energy
entropy of reaction
Correct answer is A
No explanation has been provided for this answer.
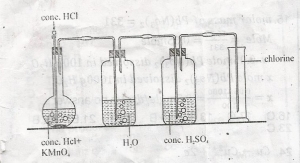
In the diagram, the function of the concentrated H2SO4 is to?
purify the gas
dry the gas
liquefty the gas
remove odour
Correct answer is B
Concentrated sulphuric acid is a very effective drying agent. The chlorine obtained as the product from the above reaction contains traces of water vapour, and hydrogen chloride (HCl) vapours. HCl vapours are removed by passing the gas through water, and the traces of water vapour are removed by passing through conc.sulphuric acid.
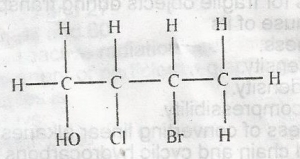
The IUPAC nomenclature of the compound above is
2-bromo-3-chlorobutanol
3-bromo-2-chlorobutanol
3-chloro-2-bromobutanol
2-chloro-3-bromobutanol
Correct answer is B
No explanation has been provided for this answer.
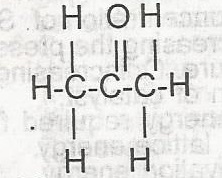
alkanone
alkanoate
alkanal
akanol
Correct answer is A
No explanation has been provided for this answer.
JAMB Subjects
Aptitude Tests