586.
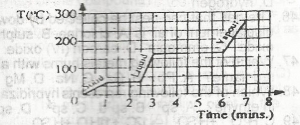

Use the graph above to answer this question.How long does it take all the solid to melt?
A.
2.5 mins
B.
6.0 mins
C.
1.0 min
D.
3.0 mins
Correct answer is A
No explanation has been provided for this answer.
587.
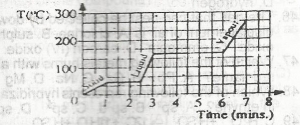

A.
125 oC
B.
150oC
C.
175oC
D.
250oC
Correct answer is B
No explanation has been provided for this answer.
588.
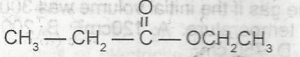
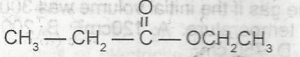
A.
ether
B.
ester
C.
alkanal
D.
alkanol
Correct answer is B
No explanation has been provided for this answer.
589.
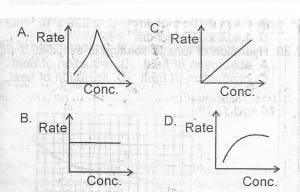

Use the option above to answer this question. The graph that describes a zero order reaction is
A.
A
B.
B
C.
C
D.
D
Correct answer is B
No explanation has been provided for this answer.
590.
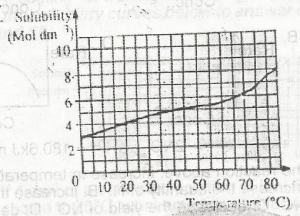

A.
0.750 mole
B.
0.950 mole
C.
2.375 moles
D.
4.750 moles
Correct answer is A
No explanation has been provided for this answer.
JAMB Subjects
Aptitude Tests