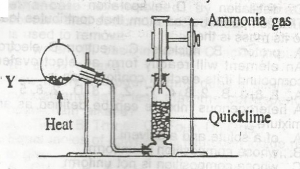
In the diagram above, mixture Y is
NH4NO3(s) + CaSO4(s),/sub>
NH4CL(s) + NaNO2(s)
NH4NO2(s) + Na2SO4(s)
NH4CL(s) + Ca(OH)2(s)
Correct answer is D
No explanation has been provided for this answer.
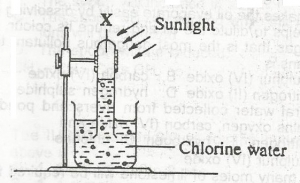
In the diagram above, gas X is
hydrogen
chlorine
oxygen
hydrogen chloride
Correct answer is C
No explanation has been provided for this answer.
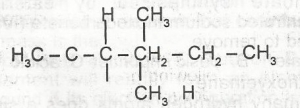
The IUPAC nomenclature of the compound above is
2-ethylpentane
3,4-dimethylhexane
2,3-dimethylhexane
2-ethylhexane
Correct answer is B
No explanation has been provided for this answer.
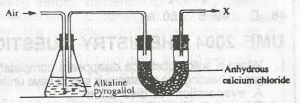
In the experiment above, X is a mixture of nitrogen, carbon (IV) oxide and
water
impurities
inert gas
oxygen
Correct answer is C
No explanation has been provided for this answer.
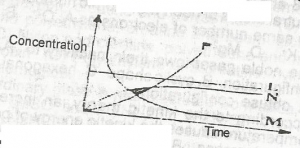
L
M
N
P
Correct answer is B
No explanation has been provided for this answer.
JAMB Subjects
Aptitude Tests