An experiment test of the Law of conservation of Mass is ...
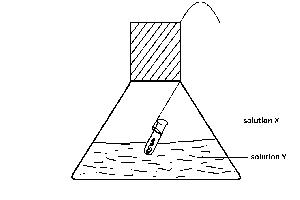
An experiment test of the Law of conservation of Mass is illustrated in the diagram. In practice, the flask is weighed before and after reaction between solutions X and Y. Which of the following pairs of solutions will be unsuitable for the experiments
X = hydrochloric acid; Y = silver nitrate
X = barium chloride; Y = dilute sulphuric acid
X = hydrochloric acid; Y = sodium hydroxide
X = hydrochloric acid; Y = lead nitrate
X = hydrochloric acid; Y = sodium carbonate
Correct answer is E
No explanation has been provided for this answer.
Similar Questions
The number of atoms in 1.2g of carbon - 12 isotope is ...
Electrons enter into orbitals in order of increasing energy as exemplified by? ...
Which of the following chloride would exhibit the least ionic character? ...
Radon is used as a tracer in medical research because it ...
From the diagram above, an ideal gas is represented by...
The gas that gives brown colouration in brown ring test is ...
Consider the reaction represented by the following equation: H 2(g) + 12 O(g) → H 2O(...
What is the total number of shared pair of electrons in the compound above? ...