A primary alkanol has a molecular mass of 60. What is the...
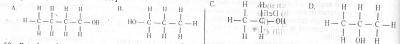
A primary alkanol has a molecular mass of 60. What is the structural formula of the compound? [C= 12.0, H= 1.0, O= 16.0)
A.
A
B.
B
C.
C
D.
D
Correct answer is B
Alkanols have the formula CnH2n+1OH.
⇒ 12n+(1×(2n+1))+16.0+1.0=60
= (14n +18 = 60\)
n=60−1814=4214=3
Hence, the alkanol is gotten by putting n=3,
=C3H7OH