In the experiment above, X could be a solution of
...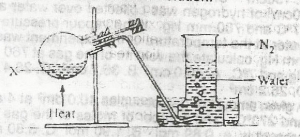
In the experiment above, X could be a solution of
sodium trioxonitrate (V) and ammonium chloride
sodium trioxonitrate (lll) and ammonium chloride
lead (ll) trioxonitrate (V) and copper turnings
potassium trioxonitrate (V) and copper turnings
Correct answer is B
Nitrogen is prepared in the laboratory by heating an equimolar aqueous solution of ammonium chloride and sodium nitrite. The ammonium nitrite formed as a result of a double decomposition reaction, decomposes to form nitrogen gas.
NH4Cl(aq)+NaNO2(aq)→NH4NO2(aq)+NaCl(aq)
NH4NO2(aq)→N2(g)+2H2O(l).
(source: understanding chemistry by G O Ojukuku)
Similar Questions
Which of the following is used as an anaesthetic?...
Propane carbon(IV)oxide diffuse at the same rate because [H = 1.00, C = 12.0, O = 16.0] ...
Which of the following metals is NOT extracted by electrolysis? ...
Which of the following compounds is a soapless detergent? ...
The by-product of fermentation of sugar is ...
What is the total number of shared pair of electrons in the compound above? ...
The percentage by mass of calcium in Ca(OCI)2 is [Ca = 40.0; CI = 35.5; O = 16.0] ...