The diagram above illustrates a conical flask containing ...
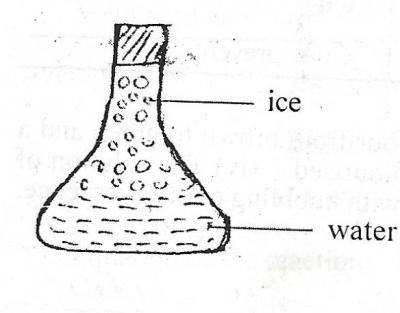
The diagram above illustrates a conical flask containing water and ice. Which of the following is correct about the diagram?
The water is at lower temperature than the ice
Energy is absorbed when the ice changes to water
Energy is released when the ice changes to water
The water molecules vibrate about a fixed point
Correct answer is B
Heat energy near the ice gets absorbed into the ice. The energy breaks the bonds of the ice, causing it to melt into liquid water. On other words, when ice melts, the energy gets absorbed into the water.
Similar Questions
On exposing palm wine to air for some days, it becomes sour owing to the conversion of ...
Zn(s) + CuSO4(aq) → ZnSO4(aq) + Cu(s) In the reaction above, the oxidizing agent is...
CO(g) + H2O(g) → CO2(g) + H2(g) From the reaction above, calculate the standard enthalpies of for...
What is the atomic number of Ruthenium? ...
Fluorine does not occur in the free state in nature because ...
When alkanols react with sodium, the ga evolved is ...
When excess ethanol is heated to 145oC in the presence of concentrated H2SO4, the product is...