The figure above shows the electrolysis of molten sodium ...
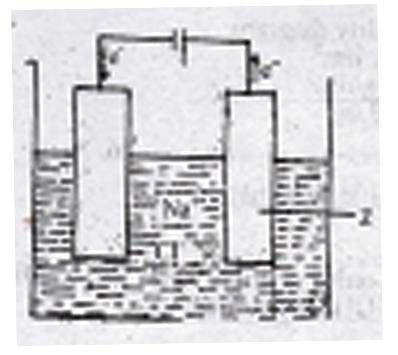
The figure above shows the electrolysis of molten sodium chloride. Z is the
anode where the Cl− ions are oxidized
cathode where the Cl− ions are reduced
anode where the Na+ ions are reduced
cathode where the Na+ ions are oxidized
Correct answer is A
During the electrolysis of brine, the chlorine ions are discharged at the anode and are oxidized. While at the cathode Hydrogen ion is discharged.
Similar Questions
The molar mass of a compound X is 56. its empirical formula is CH2. X is ...
If the cost of electricity required to discharge 10g of an ion X3+ is N20.00, how much would ...
The decolourization of purple colour of tetraoxomanganate (VII) ion is a test for ...
Vulcanization involves the removal of ...
The pollutant that contributes to the depletion of the ozone layer is ...
The heat energy produced when the human body metabolises 1 gram of fat is ...