In the shown experiment (Fig. 1) the litmus paper will in...
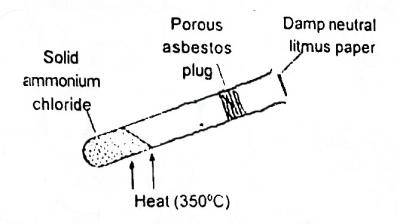
In the shown experiment (Fig. 1) the litmus paper will initially
be bleached
turn green
turn red
turn blue
Correct answer is D
NH4Cl→NH3+HCl. rate of diffusion of substances depends too on their molar mass, the smaller the molar mass of a substance the faster the rate of diffusion of that substance. so the litmus paper initially turns blue because ammonia will get to it first since it diffuses faster than HCl.
Similar Questions
The chlorinated alkane often used industrially to remove grease is ...
The shape of ammonia molecules is ...
A positive reaction to Fehling's test indicates the presence of ...
The heat required to raise the temperature of the body by 1k is called? ...
A spot of oil paint on a shirt can best be removed using? ...
Copper (II) ions are able to participate in co-ordinate covalent bonding because they ...
Which compound will have the lowest boiling point? ...
CuO(s) + H2(g) ↔ Cu(s) + H2O(g) What is the effect of increasing the pressure on the...
The high boiling point of hydrogen fluoride can be explained in terms of? ...