The electron configuration of 26Fe3+ is?
...The electron configuration of 26Fe3+ is?
[Ar]4s23d6
[AR]4s23d3
[AR]4s13d4
[AR]4s03d5
Correct answer is D
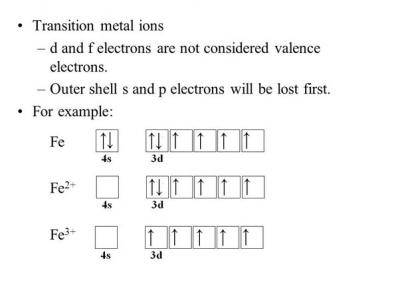
The neutral Iron atom has the electric configuration: 1s2 2s2 2p6 3s2 3p6 4s2 3d6.to write the electronic config. of Fe3+ we have to subtract 3 electrons from the outermost shell which is 4s and 3d orbitals. Thus we obtain the electronic configuration for Fe3+ as:
1s2 2s2 2p6 3s2 3p6 4s0 3d5
OR
[AR] 4s0 3d5.
Similar Questions
If an equilibrium reaction has ∆H < O, the reaction will proceed favorably in the forward react...
The solubility curve shows the variation of solute concentration with ...
Which of the following will displace copper from a solution of copper (ll) salt? ...
In the production of soap, concentrated sodium chloride solution is added to ...
What is the maximum number of electrons that can occupy the second energy level (n=2)? ...
What is the atomic number of Iridium? ...
As the difference in electronegativity between bonded atoms increases, polarity of the bond? ...
The gas used in the manufacture of vanaspati from vegetable oil is ...