In the diagram above, the gas produced is?
...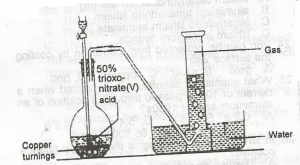
In the diagram above, the gas produced is?
NO
NO2
N2O
N2O4
Correct answer is B
In this laboratory preparation, 50% trioxonitrate (V) acid is reacted with copper turnings to liberate nitrogen (II) oxide ( NO ) that is collected by downward delivery.
Cu+\(4HNO_3 →Cu(NO_3)_2+2NO_2+2H_2O\)
Similar Questions
Consider the following reaction equation: CaO + SiO\(_{2}\) ------> CaSiO\(_{3}\) Silicon(IV) o...
When air is passed through alkaline pyrogallol and then over quicklime, the only gases left are? ...
A visible change is observed when a strip of iron is placed in an aqueous solution of ...
Which of these salts will produce its metal, oxygen and nitrogen (IV) oxide on heating? ...
Sodium reacts vigorously with water to produce ...
Which of the following statement is correct? The solubility of ...