Choose the correct option from the structure above
...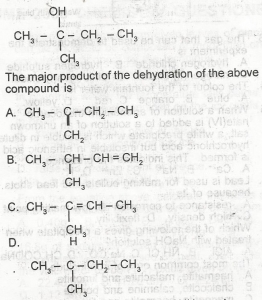
Choose the correct option from the structure above
A.
A
B.
B
C.
C
D.
D
Correct answer is C
No explanation has been provided for this answer.
Similar Questions
Biotechnology is applied in the following except ...
What is the volume of oxygen required to burn complete 45 cm3 of methane at s.t.p? ...
The diagram above represents the formation of ...
How many grams of bromine will be required to completely react with 10 g of propyne? (C = 12, H =...
Which of the following process does not take place in domestic water treatment? ...