2HCL(ag) + CaCO3(ag) → CaCL2(ag) + H2O(1) + CO from ...
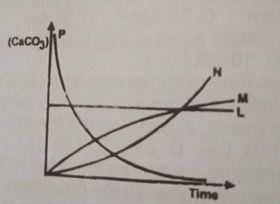
2HCL(ag) + CaCO3(ag) → CaCL2(ag) + H2O(1) + CO from the reaction above, which of the following curves represents the consumption of calcium trioxocarbonate (IV) as dilute HCL is added to it?
L
M
N
P
Correct answer is D
The concentration of the CaCO3 decreases as reaction time decreases. therefore option D is correct.
Similar Questions
The compound above contains ...
The relationship between equilibrium constant and free energy change is represented as ...
Aluminium is used in the manufacture or aircraft because it ...
Consider the following equilibrium system: 2SO2(g) + O2(g) ⇌ 2SO3(g) The ad...
Nuclear reactions can be used in the following except ...
What are the possible oxidation numbers of an element if its atomic number is 17? ...
Which of the compounds will leave a metal residue when heated? ...
A salt which loses mass when exposed to air is...
The type of energy changes that accompany the mixing of a strong acid to a strong base is ...