The above diagram gives the potential energy profile of the ...
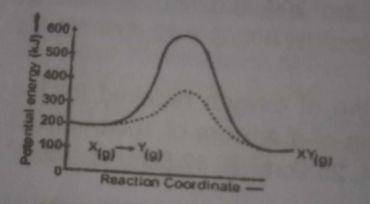
The above diagram gives the potential energy profile of the catalyst and uncatalysed reactions of X(g) + Y(g) → XY(g). Deduce the respective activation energies in KJ of the catalysed and uncatalysed reverse reactions: XY(g) → X(g) + Y(g)
A.
300, 500
B.
500, 300
C.
-300, -500
D.
-500, -300
Correct answer is C
The uncatalysed reverse reaction = 100 - 400 = -300 The calalysed reverse reaction = 100 - 600 = - 500
Similar Questions
Which of the following ions has an electron configuration different from the other? ...
When chlorine is passed into water and subsequently exposed to sunlight, the gas evolved is? ...
What is the chemical symbol for Iodine? ...
The rate of chemical reaction of solids are not affected by ...
Which of the following statements is NOT true of carbon monoxide?...
A metal that forms soluble trioxosulphate (IV) ion is ...
The properties of a good primary standard solution include the following except? ...
The iron (III) oxide impurity in bauxite can be removed by? ...