The diagram shown above represents the solubility curves of ...
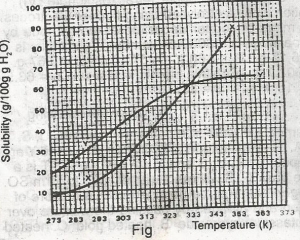
The diagram shown above represents the solubility curves of two salts, X and Y, in water, use this diagram to answer the question. If the molar mass of X is 36 g, the number of moles of X dissolved at 343 K is
A.
0.2 moles
B.
0.7 moles
C.
1.5 moles
D.
2.0 moles
E.
3.0 moles
Correct answer is D
The molar mass of x = 36g. At 343k, 72g of x dissolved
Molarity of x = 7236 = 2.00moles.
Similar Questions
The pH of water is 7. What is the pH of orange juice? ...
What is the chemical symbol for Oganesson? ...
What is the atomic number of Meitnerium? ...
The basic particles from which matter could be made up of are as follow, except? ...
A salt which loses mass when exposed to air is...
What is the atomic number of Rubidium? ...
Consecutive members of an alkane homologous serires differ by ...
What is CaHb in the following equation? C a H b + 5O2 → 3CO 2 + 4H2O...