The diagram shown above represents the solubility curves of ...
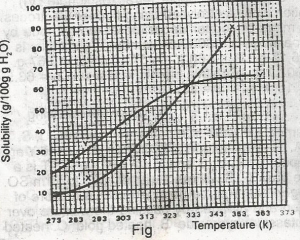
The diagram shown above represents the solubility curves of two salts, X and Y, in water, use this diagram to answer the question. At room temperature (300 K)
A.
Y is twice as soluble as X
B.
X is twice as soluble as Y
C.
X and Y are soluble to the same extent
D.
X is three times as soluble as Y
E.
Y is three times as soluble as X
Correct answer is A
No explanation has been provided for this answer.
Similar Questions
The gas that is the most dangerous pollutant to human is ...
The alkanol represented by the structure above is ...
The electron configuration of two elements with similar chemical properties are represented by? ...
What process is illustrated below? S(s) ⇌H2O⇌ S (aq) ...
Soluble salts can be prepared by the following methods except ...