The solubility curve of a solid X (molecular mass = 160) is ...
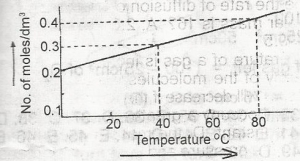
The solubility curve of a solid X (molecular mass = 160) is as show above. 1,000 cm3 of a saturated solution of X at 80°C is cooled to 40°C . The weight of X crystalized out would be
A.
10 g
B.
12 g
C.
14 g
D.
16 g
E.
18 g
Correct answer is D
At 80°C, we have 1000cm3=0.4mol/cm3
40°C = 0.3mol/cm3
hence, the loss in concentration = 0.1mol/cm3
= 0.1×160=16g
Similar Questions
Polyatomicity is illustrated by molecules of ...
Which of the following electron configurations represents that of an atom in its ground state? ...
A balanced chemical equation obeys the law of ...
The boiling point of water is higher than the boiling point of ethanol because ...
If the volume of a gas at 35°C is 60cm3, what would be its volume in cm3 at 273k?...