What would happen if solution Y is more concentrated than...
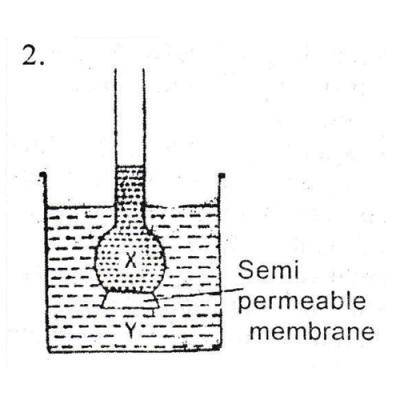
What would happen if solution Y is more concentrated than solution X in fig 2
The level of X would rise, Y would fall
The level of X would rise, Y would rise
The level of X and Y stands the same
The level of Y would rise, X would fall
Correct answer is D
Osmosis is the spontaneous net movement of solvent molecules through a selectively permeable membrane into a region of higher solute concentration, in the direction that tends to equalize the solute concentrations on the two sides. Therefore, the level of Y (region of higher concentration) would rise, X (region of lower concentration) would fall to equalize the solute concentrations on both sides.
Similar Questions
Which of the following actions is NOT a voluntary action? ...
Which of the following relationships illustrates competition? ...
Which of the following is NOT a characteristic of digestive enzymes? ...
The transmission of impulses along a nerve fibre is characterized by ...
The adaptive importance of the nuptial flight from termite colonies is to ...
The thyroid gland is found at the base of the ...
Growing radicles of seedlings are ...