law of multiple proportions
law of chemical proportions
law of simple proportions
law of conservation of mass
Correct answer is A
The law of multiple proportion states that when element A and B combine to form more than one chemical compound then the various masses of sample A react with a fixed mass of sample B are in a simple multiple ratio.
The knowledge of half-life can be used to
Create an element
Detect an element
To combine elements
Irradiate an element
Correct answer is B
Half-life, in radioactivity, the interval of time required for one-half of the atomic nuclei of a radioactive sample to decay (change spontaneously into other nuclear species by emitting particles and energy), or, equivalently, the time interval required for the number of disintegrations per second of a radioactive material to decrease by one-half
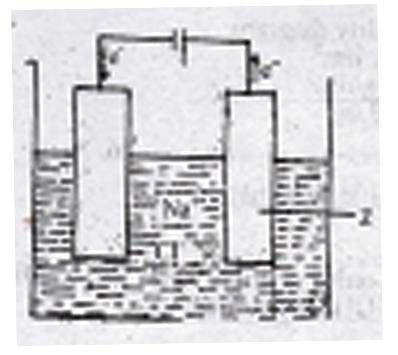
The figure above shows the electrolysis of molten sodium chloride. Z is the
anode where the Cl\(^-\) ions are oxidized
cathode where the Cl\(^-\) ions are reduced
anode where the Na\(^+\) ions are reduced
cathode where the Na\(^+\) ions are oxidized
Correct answer is A
During the electrolysis of brine, the chlorine ions are discharged at the anode and are oxidized. While at the cathode Hydrogen ion is discharged.
Which of the following produces relatively few ions in solution?
NaOH
Ca(OH)\(_2\)
KOH
AI(OH)\(_3\)
Correct answer is D
Aluminium hydroxide is not soluble in water because it is polymeric and it also has high lattice energy and therefore it is hard to break bonds in this compound. So, water cannot dissolve such types of compounds.
Al(OH)\(_3\) is not soluble in water
The choice of method for extracting a metal from its ores depends on the
strength of the core
position of the metal in the electrochemical series
source of the core
position of the metal in the periodic table
Correct answer is B
The method used to extract a metal from its ore depends upon the stability of its compound in the ore, which in turn depends upon the reactivity of the metal:
• The oxides of very reactive metals, such as aluminum, form stable oxides and other compounds. A lot of energy is needed to reduce them to extract the metal.
• The oxides of lesser reactive metals, such as iron, form less stable oxides and other compounds. Relatively little energy is needed to reduce them to extract the metal.
So, the method of extraction of a metal from its ore depends on the metal's position in the reactivity series.
JAMB Subjects
Aptitude Tests